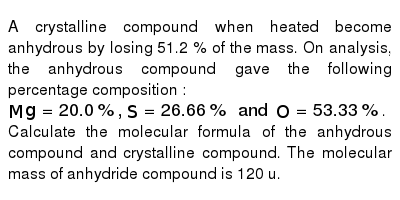
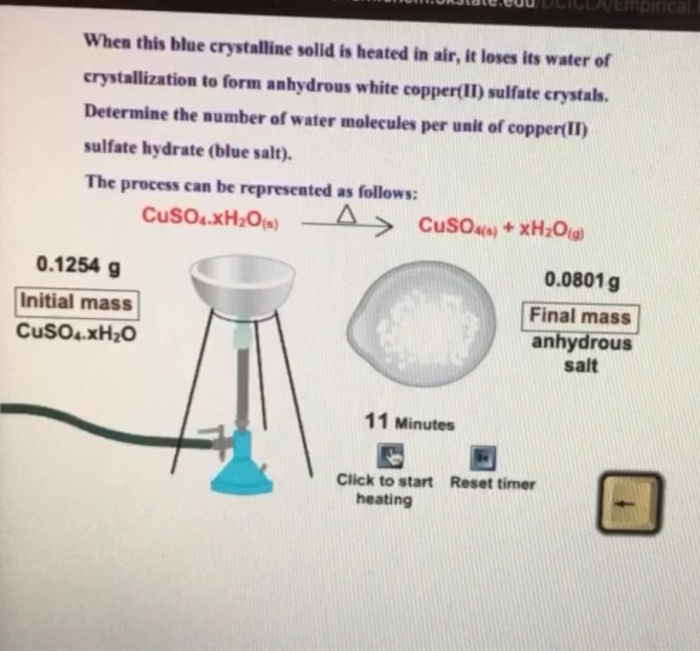
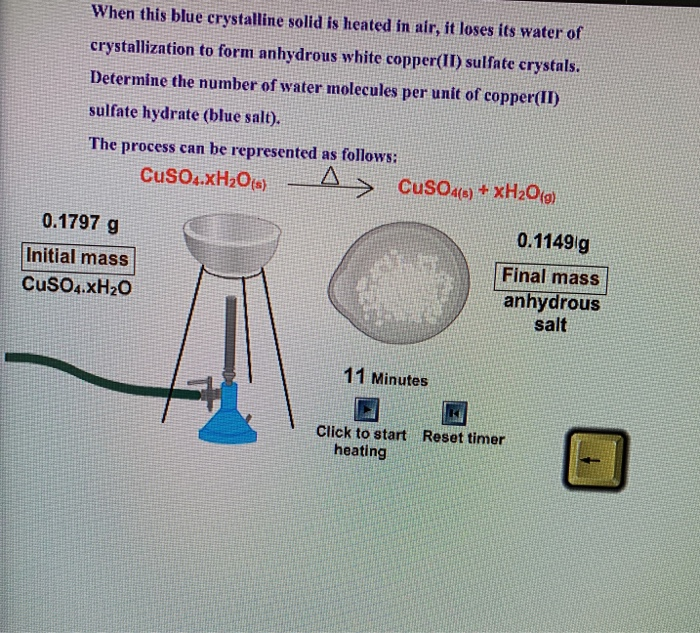
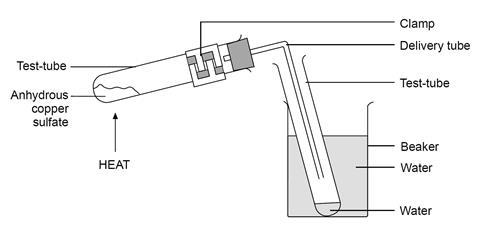
A' is a blue coloured crystalline salt. On heating it loses blue colour and to give 'B' - Sarthaks eConnect | Largest Online Education Community

The metal salt A is in blue colour. When salt A is heated strongly over a burner then a substance B present in it is eliminated and a white powder C is
NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment | Heritage Science | Full Text

Question Identify the substances.P, Q, R, S and T in each case based on the information given below: (i) The deliquescent salt P, turns yellow on dissolving in water, and gives a
NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment | Heritage Science | Full Text

The metal salt A is blue in color. When salt A is heated strongly over a burner, then a substance B - Brainly.in
What is meant by water of crystallisation? Explain that the crystalline salts contain water of crystallisation. - Sarthaks eConnect | Largest Online Education Community