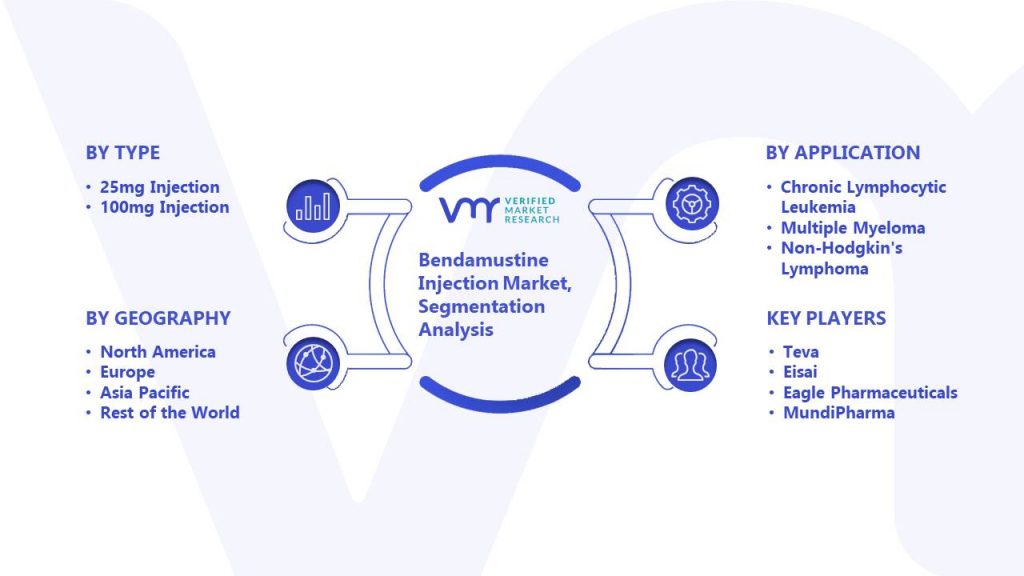
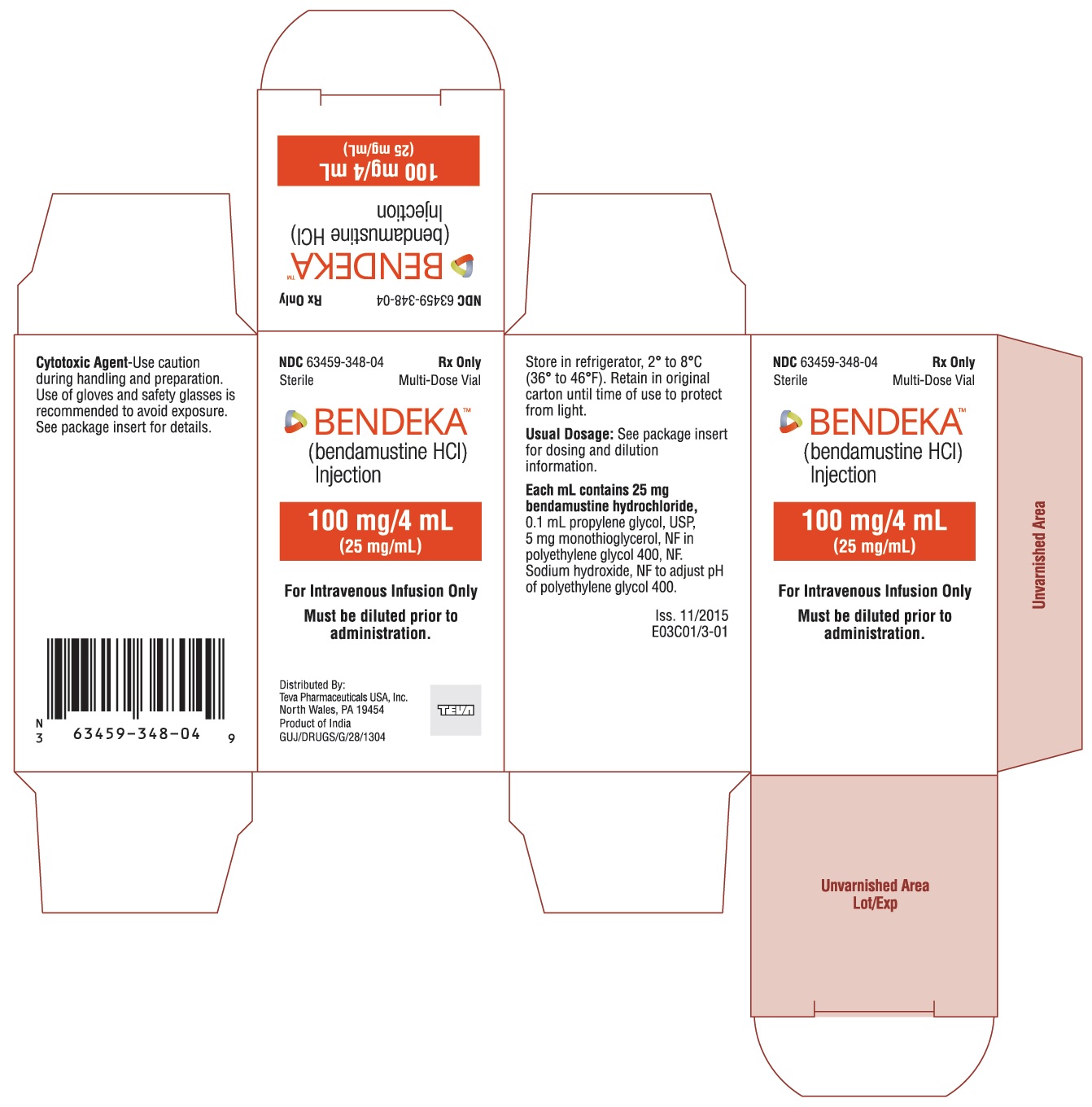
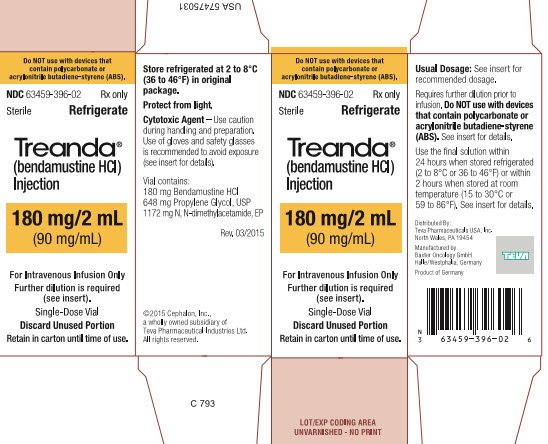
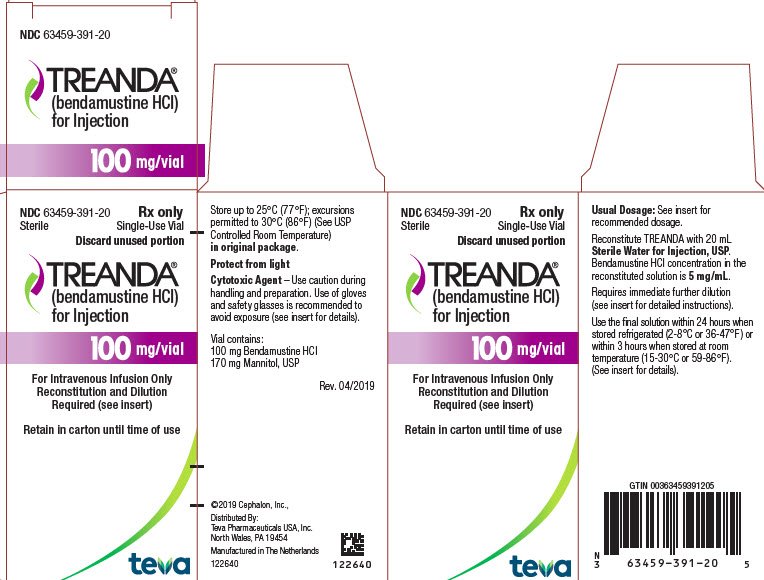
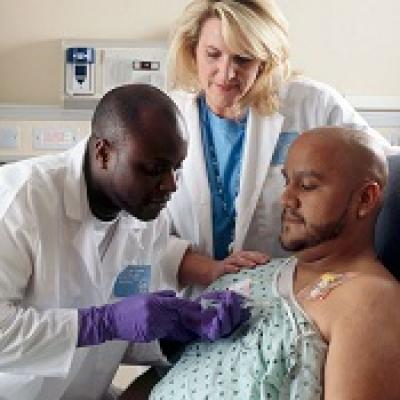
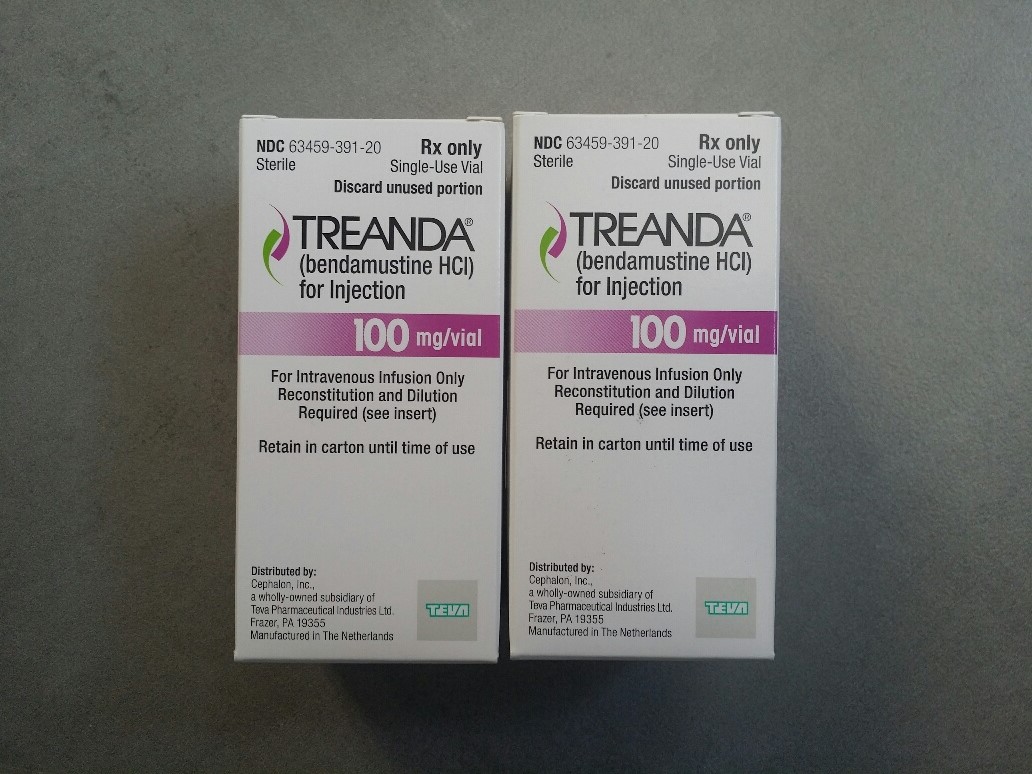
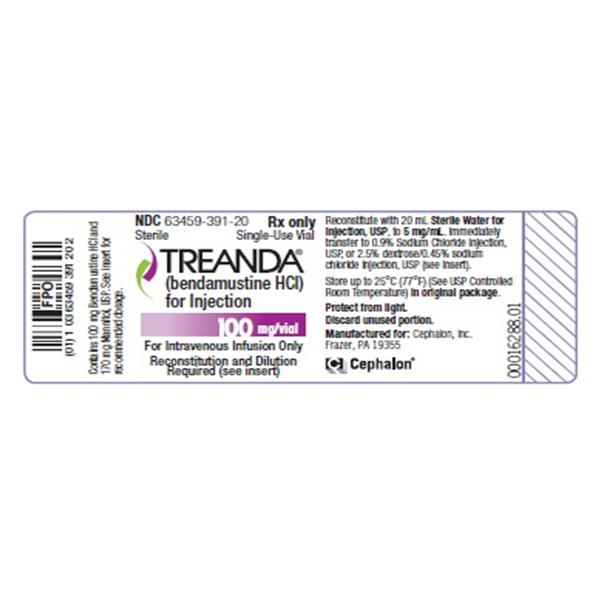
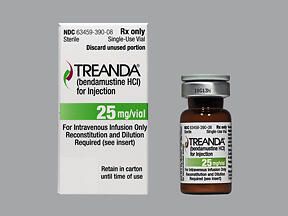
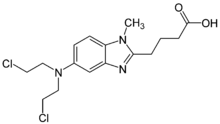
BENDEKA (BENDAMUSTINE HCI) INJECTION Trademark of TEVA PHARMACEUTICALS INTERNATIONAL GMBH - Registration Number 5014704 - Serial Number 86826912 :: Justia Trademarks

Teva, chasing Amgen's Aimovig, scores much-needed FDA approval for migraine drug Ajovy | FiercePharma